Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nomura, Mikihiro*; Kodaira, Takahide*; Ikeda, Ayumi*; Naka, Yasuhito*; Nishijima, Haruyuki*; Imabayashi, Shinichiro*; Sawada, Shinichi*; Yamaki, Tetsuya*; Tanaka, Nobuyuki; Kubo, Shinji
Journal of Chemical Engineering of Japan, 51(9), p.726 - 731, 2018/09
Times Cited Count:3 Percentile:12.96(Engineering, Chemical)Thermochemical hydrogen production by the iodine-sulfur process decomposes water into hydrogen and oxygen by combining the chemical reactions of iodine and sulfur. Two types of acids are produced through the Bunsen reaction. To improve the performance of this reaction, ion-exchange membranes for the membrane Bunsen reaction should be developed. In the present study, a cation-exchange membrane was prepared by using a radiation-graft polymerization method. It was found that a divinylbenzene crosslinking procedure was very effective in reducing water permeation through the membrane, and the membrane Bunsen reaction was successfully carried out by using the developed crosslinked membrane. Therefore, the developed crosslinked membrane is a potential candidate for cation-exchange membranes for the membrane Bunsen reaction.
Nakahara, Masaumi; Sano, Yuichi; Nomura, Kazunori; Takeuchi, Masayuki
Journal of Chemical Engineering of Japan, 51(3), p.237 - 242, 2018/03
Times Cited Count:2 Percentile:8.98(Engineering, Chemical)For evaluating the Pu partitioning behavior under the condition of high Pu concentration in the feed solution by the acid split method, the counter current experiment was carried out. The Pu content in the U/Pu product was 1.51 times higher than that in the feed solution. In the Pu partitioning section, Pu polymerization and third phase formation were observed, and the operation of centrifugal contactors was stable.
Uchiyama, Shoichiro*; Ishihara, Ryo*; Umeno, Daisuke*; Saito, Kyoichi*; Yamada, Shinsuke*; Hirota, Hideyuki*; Asai, Shiho
Journal of Chemical Engineering of Japan, 46(6), p.414 - 419, 2013/06
Times Cited Count:7 Percentile:25.94(Engineering, Chemical)Nakahara, Masaumi; Kaji, Naoya; Yano, Kimihiko; Shibata, Atsuhiro; Takeuchi, Masayuki; Okano, Masanori; Kuno, Takehiko
Journal of Chemical Engineering of Japan, 46(1), p.56 - 62, 2013/01
Times Cited Count:1 Percentile:6.3(Engineering, Chemical)The influence of HNO concentration in the solution on the formation of Cs
concentration in the solution on the formation of Cs Pu(NO
Pu(NO )
) was evaluated in the U crystallization process. The solubility of Cs
was evaluated in the U crystallization process. The solubility of Cs Pu(NO
Pu(NO )
) in a uranyl nitrate solution was found to decrease with increasing HNO
in a uranyl nitrate solution was found to decrease with increasing HNO concentration in the solution. In the U crystallization experiments with the dissolver solution of irradiated fast reactor fuel, Cs
concentration in the solution. In the U crystallization experiments with the dissolver solution of irradiated fast reactor fuel, Cs Pu(NO
Pu(NO )
) formed with 6.5 mol/dm
formed with 6.5 mol/dm HNO
HNO concentration in the mother liquor, and the decontamination factor of Cs for the uranyl nitrate hexahydrate crystals was low. Meanwhile, Cs
concentration in the mother liquor, and the decontamination factor of Cs for the uranyl nitrate hexahydrate crystals was low. Meanwhile, Cs Pu(NO
Pu(NO )
) did not precipitate with uranyl nitrate hexahydrate crystals under the condition of 4.0 mol/dm
did not precipitate with uranyl nitrate hexahydrate crystals under the condition of 4.0 mol/dm HNO
HNO concentration in the mother liquor, and Cs could be separated from the uranyl nitrate hexahydrate crystals.
concentration in the mother liquor, and Cs could be separated from the uranyl nitrate hexahydrate crystals.
Nakahara, Masaumi; Koma, Yoshikazu
Journal of Chemical Engineering of Japan, 44(5), p.313 - 320, 2011/05
Times Cited Count:4 Percentile:17.76(Engineering, Chemical)The influence of the HNO concentration in the feed and scrubbing solutions on the behavior of Np was evaluated experimentally and found to be co-extracted into the tri-
concentration in the feed and scrubbing solutions on the behavior of Np was evaluated experimentally and found to be co-extracted into the tri- -butylphosphate with U and Pu. Almost all the Np in a 4.9 mol/dm
-butylphosphate with U and Pu. Almost all the Np in a 4.9 mol/dm HNO
HNO feed solution was recovered with U and Pu, based on the experimental flow sheet with double scrubbing solutions of 9 and 1 mol/dm
feed solution was recovered with U and Pu, based on the experimental flow sheet with double scrubbing solutions of 9 and 1 mol/dm HNO
HNO . The experimental results showed a large contribution from the HNO
. The experimental results showed a large contribution from the HNO concentration in the feed solution and at the extraction section to Np(V) oxidation. On the other hand, calculation results showed that high HNO
concentration in the feed solution and at the extraction section to Np(V) oxidation. On the other hand, calculation results showed that high HNO concentrations in the feed solution tended to leak Np into the raffinate.
concentrations in the feed solution tended to leak Np into the raffinate.
Kubota, Fukiko*; Shimobori, Yosuke*; Baba, Yuzo*; Koyanagi, Yusuke*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Journal of Chemical Engineering of Japan, 44(5), p.307 - 312, 2011/05
Times Cited Count:57 Percentile:82.59(Engineering, Chemical)The application of ionic liquids as alternatives to conventional organic solvents in extraction processes has been actively investigated. A crucial step towards the practical use of ionic liquids is the development of extractants that work effectively within these new media. In the present study, the extraction separation of rare earth metals into ionic liquids, 1-butyl, 1-octyl, and l-dodecyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C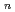 min][Tf
min][Tf N],
N],  = 4, 8, 12), was performed using a novel extractant,
= 4, 8, 12), was performed using a novel extractant,  ,
, -dioctyldiglycol amic acid (DODGAA). Quantitative extraction of metal ions such as Y
-dioctyldiglycol amic acid (DODGAA). Quantitative extraction of metal ions such as Y and Eu
and Eu was selectively achieved in the presence of the base metal ion Zn
was selectively achieved in the presence of the base metal ion Zn , which was not extracted at all under the present experimental conditions. The extraction efficiency was enhanced for the shorter-alkyl-chain imidazolium ionic liquid [C
, which was not extracted at all under the present experimental conditions. The extraction efficiency was enhanced for the shorter-alkyl-chain imidazolium ionic liquid [C mim] [Tf
mim] [Tf N] compared to that for a conventional organic solvent system. Extraction mechanism studies elucidated that the metal extraction proceeds via proton exchange reactions between DODGAA and the metal ions in the ionic liquid (the same mechanism as in the conventional organic solvent). The stripping reaction, or recovery, of the metal ions from the extracting phase was readily accomplished with an acid solution such as nitric acid.
N] compared to that for a conventional organic solvent system. Extraction mechanism studies elucidated that the metal extraction proceeds via proton exchange reactions between DODGAA and the metal ions in the ionic liquid (the same mechanism as in the conventional organic solvent). The stripping reaction, or recovery, of the metal ions from the extracting phase was readily accomplished with an acid solution such as nitric acid.
Ishii, Katsunori; Suzuki, Masahiro; Yamamoto, Takuma; Kihara, Yoshiyuki; Kato, Yoshiyuki; Kurita, Tsutomu; Yoshimoto, Katsunobu; Yasuda, Masatoshi*; Matsusaka, Shuji*
Journal of Chemical Engineering of Japan, 42(5), p.319 - 324, 2009/05
Times Cited Count:7 Percentile:29.69(Engineering, Chemical)The flowability of coarse particles has been experimentally investigated using the vibrating tube method, to evaluate the applicability of this method to MOX (mixed oxide of PuO and UO
and UO ) particles which are nuclear fuel used for electric power production. Five sizes of non-radioactive model particles, smaller than 850 micrometers, made of ZrO
) particles which are nuclear fuel used for electric power production. Five sizes of non-radioactive model particles, smaller than 850 micrometers, made of ZrO were prepared, and the experiments were carried out using vibrating tubes with an outlet diameter from 2 to 4 mm. The outlet diameter significantly affected the flowability measurements. When using the tube with a 4-mm-outlet diameter, the flowability of all the model particles was successfully measured. The inclination angle of the tube, also, affected the flowability measurements. From the advantages of high sensitivity, short measurement time, simple structure, and easy operation, the vibrating tube method is expected to be applied to the remote flowability measurement of the MOX particles.
were prepared, and the experiments were carried out using vibrating tubes with an outlet diameter from 2 to 4 mm. The outlet diameter significantly affected the flowability measurements. When using the tube with a 4-mm-outlet diameter, the flowability of all the model particles was successfully measured. The inclination angle of the tube, also, affected the flowability measurements. From the advantages of high sensitivity, short measurement time, simple structure, and easy operation, the vibrating tube method is expected to be applied to the remote flowability measurement of the MOX particles.
Shimada, Akihiko; Hakoda, Teruyuki; Kojima, Takuji
Journal of Chemical Engineering of Japan, 39(9), p.980 - 986, 2006/09
Times Cited Count:0 Percentile:0.01(Engineering, Chemical)Electron beam (EB) suitable for decomposition of dilute organic pollutants in high-flow rate air was applied to the treatment of formaldehyde (HCHO)/air mixture for purification in the clean room after sterilization. Air mixtures containing 90-1230 ppmv of HCHO with and without 30-340 ppmv of methanol (CH OH), which are commonly used for sterilization, were irradiated by EB to the absorbed doses of 5-80 kGy (=kJ/kg). The dose required for decreasing initial HCHO concentration to 10 ppmv was examined as a function of initial HCHO concentration (ppmv) in the air mixture ([HCHO]
OH), which are commonly used for sterilization, were irradiated by EB to the absorbed doses of 5-80 kGy (=kJ/kg). The dose required for decreasing initial HCHO concentration to 10 ppmv was examined as a function of initial HCHO concentration (ppmv) in the air mixture ([HCHO]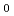 ) with and without CH
) with and without CH OH. The following relations were obtained:
OH. The following relations were obtained:  =-4.2
=-4.2 10
10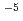 [HCHO]
[HCHO]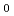
 +1.2
+1.2 10
10 [HCHO]
[HCHO]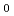 -1.2 and
-1.2 and  =-3.8
=-3.8 10
10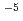 [HCHO]
[HCHO]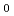
 +1.0
+1.0 10
10 [HCHO]
[HCHO]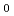 -1.0, respectively, where
-1.0, respectively, where  (kGy) is dose. According to these results, the treatment completion time of HCHO/air mixture with CH
(kGy) is dose. According to these results, the treatment completion time of HCHO/air mixture with CH OH using EB was simulated considering the conceptual processing. This EB processing can be applicable, e.g. the clean rooms at the food packaging factories, and contributes to improve the operation efficiency of the factories.
OH using EB was simulated considering the conceptual processing. This EB processing can be applicable, e.g. the clean rooms at the food packaging factories, and contributes to improve the operation efficiency of the factories.
Kasahara, Seiji; Onuki, Kaoru; Nomura, Mikihiro*; Nakao, Shinichi*
Journal of Chemical Engineering of Japan, 39(5), p.559 - 568, 2006/05
Times Cited Count:12 Percentile:42.3(Engineering, Chemical)Sensitivity analysis of the operation parameters and the chemical properties in the thermochemical hydrogen production IS process (iodine-sulfur process) was carried out for a static flow sheet. These parameters were evaluated by hydrogen production thermal efficiency, mass flow rate or heat exchange based on heat/mass balance. The most important parameters were concentration of HI after the electro-electrodialysis (EED) cell and apparent transport number of proton of the cation exchange membrane in the EED cell. HI concentration operation should be operated carefully because the parameters for optimum thermal efficiency and for optimum flow rate and heat exchange were different. For the chemical properties, composition at the inlet of the HI decomposition procedure and HIx pseudo-azeotropic composition had great effects. HI concentration after the EED cell should be optimized for each composition. Order of priority for assessment of the operation parameters and chemical properties was determined by the evaluation.
Kasahara, Seiji; Hwang, G.*; Nakajima, Hayato; Choi, H.*; Onuki, Kaoru; Nomura, Mikihiro
Journal of Chemical Engineering of Japan, 36(7), p.887 - 899, 2003/07
Times Cited Count:67 Percentile:88.08(Engineering, Chemical)Thermal efficiency of the IS thermochemical hydrogen production process was evaluated. Sensitivities of operation conditions (HI conversion ratio, pressure and reflux ratio at HI distillation and concentration of HI after EED) and nonidealities of the process (electric energy loss in EED, loss at heat exchangers and loss of waste heat recovery as electricity) were investigated. Concentration of HI after EED had the most significant effect of 13.3 % on thermal efficiency in operation conditions. Nonidealities had importance on thermal efficiency. Thermal efficiency was 56.8 % with optimized operation conditions and no nonidealities.
Hakoda, Teruyuki; Arai, Hidehiko; Hashimoto, Shoji
Journal of Chemical Engineering of Japan, 34(10), p.1300 - 1308, 2001/10
Times Cited Count:2 Percentile:25.1(Engineering, Chemical)The continuous gas monitoring system using the mass filter (CGM-MS) was developed for the measurement of gaseous substances in air under atmospheric pressure. A capillary tube introduces the gases under atmospheric pressure into a mass filter installed in a vacuum chamber. The CGM-MS detected gaseous substances, such as sulfur dioxide, benzene and chlorobenzene, with detectable sensitivity of 0.7-1 ppmv. The monitoring system was also applied for the analysis of the products formed in electron beam irradiation of trichloroethylene (TCE) and air mixture. Dichloroacetyl chloride, carbonyl chloride (COCl ), and chlorine (Cl
), and chlorine (Cl ) were quantitatively analyzed. Trichloroethylene and the products were oxidized and completely converted into carbon dioxide, Cl
) were quantitatively analyzed. Trichloroethylene and the products were oxidized and completely converted into carbon dioxide, Cl , and hydrochloric acid at 15 kGy. Carbonyl chloride is dissolved in an alkaline solution to be automatically oxidized into CO
, and hydrochloric acid at 15 kGy. Carbonyl chloride is dissolved in an alkaline solution to be automatically oxidized into CO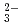 and Cl
and Cl . The combination of the irradiation and the dissolution of the irradiated gas decreased to 7 from 15 kGy for the complete oxidation of TCE and the products.
. The combination of the irradiation and the dissolution of the irradiated gas decreased to 7 from 15 kGy for the complete oxidation of TCE and the products.